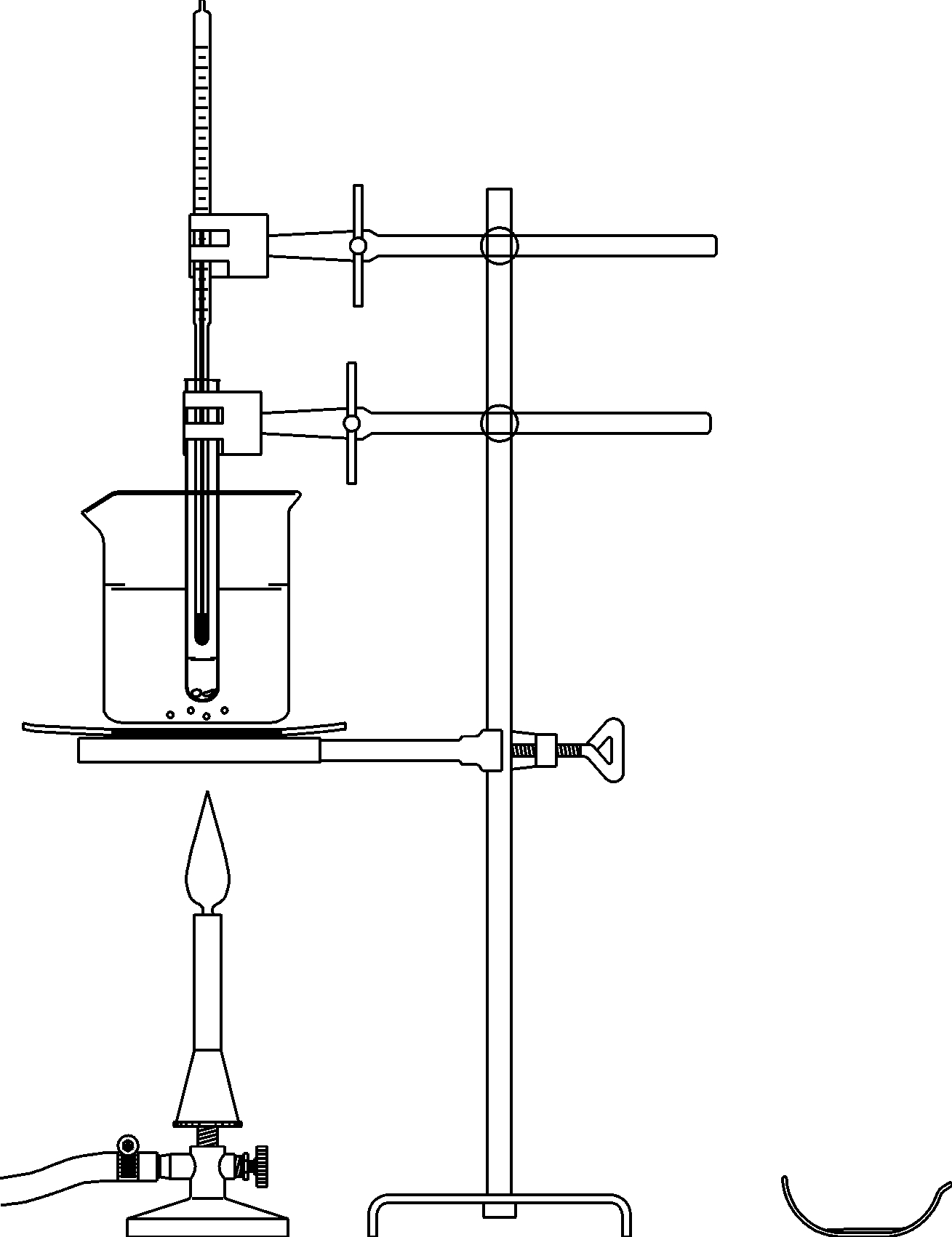
Principle
Organic compounds always contain the element carbon and the carbon atoms are combined with other carbon atoms or atoms of other elements by covalent bonds. . This is the reason why the C-C or C-H bonds are stable at normal (room) temperature.
However low heat resistance is characteristic for many organic compounds. These compounds burn or carbonize when heated over a few hundred degrees Celsius. The thermal stability of organic compounds, which is low in comparison to many salts and metals, is a result of the low polar character of C-C and C-H bonds. In this experiment the students investigate the temperature behavior of molecular (organic) substances.
Learning objectives
- Properties of organic compounds
- Thermal stability
- Many organic compounds are sensitive to heating