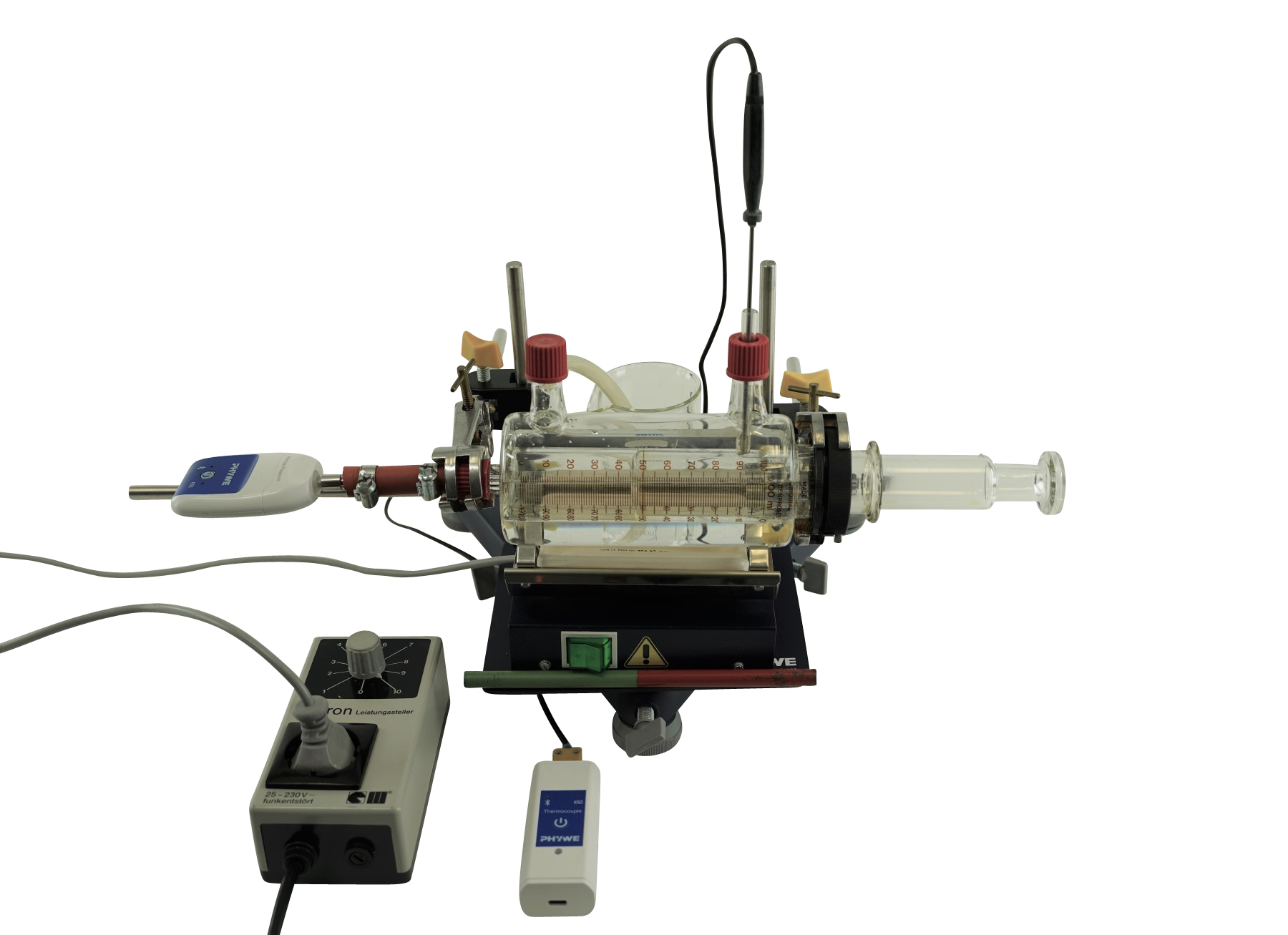
Principle
The state of a gas is determined by temperature, pressure and amount of substance. For the limiting case of ideal gases, these state variables are linked via the general equation of state. For a change of state under isochoric conditions this equation becomes Amontons' law. In this experiment it is investigated whether Amontons' law is valid for a constant amount of gas (air).
Benefits
- Unique system: All gas laws can be measured with the same setup
- Very compact setup, can be stored in the shelf and is always ready to use
- Very demonstrative: Volume is read directly at the gas syringe, temperature and pressure are measured with sensors in real-time
- Heating system is integrated in experiment setup
- Direct and versatile measurement with Cobra SMARTsense.
Tasks
- For a constant amount of gas (air) investigate the correlation of
- Volume and pressure at constant temperature (Boyle and Mariotte's law)
- Volume and temperature at constant pressure (Gay-Lussac's law)
- Pressue and temperature at constant volume (Charles' (Amontons' law))
- From the relationships obtained calculate the universal gas constant as well as the coefficient of thermal expansion, the coefficient of thermal tension, and the coefficient of cubic compressibility.
Learning objectives
- Thermal tension coefficient
- General equation of state for ideal gases
- Universal gas constant
- Amontons' law
Software included. Computer not provided.