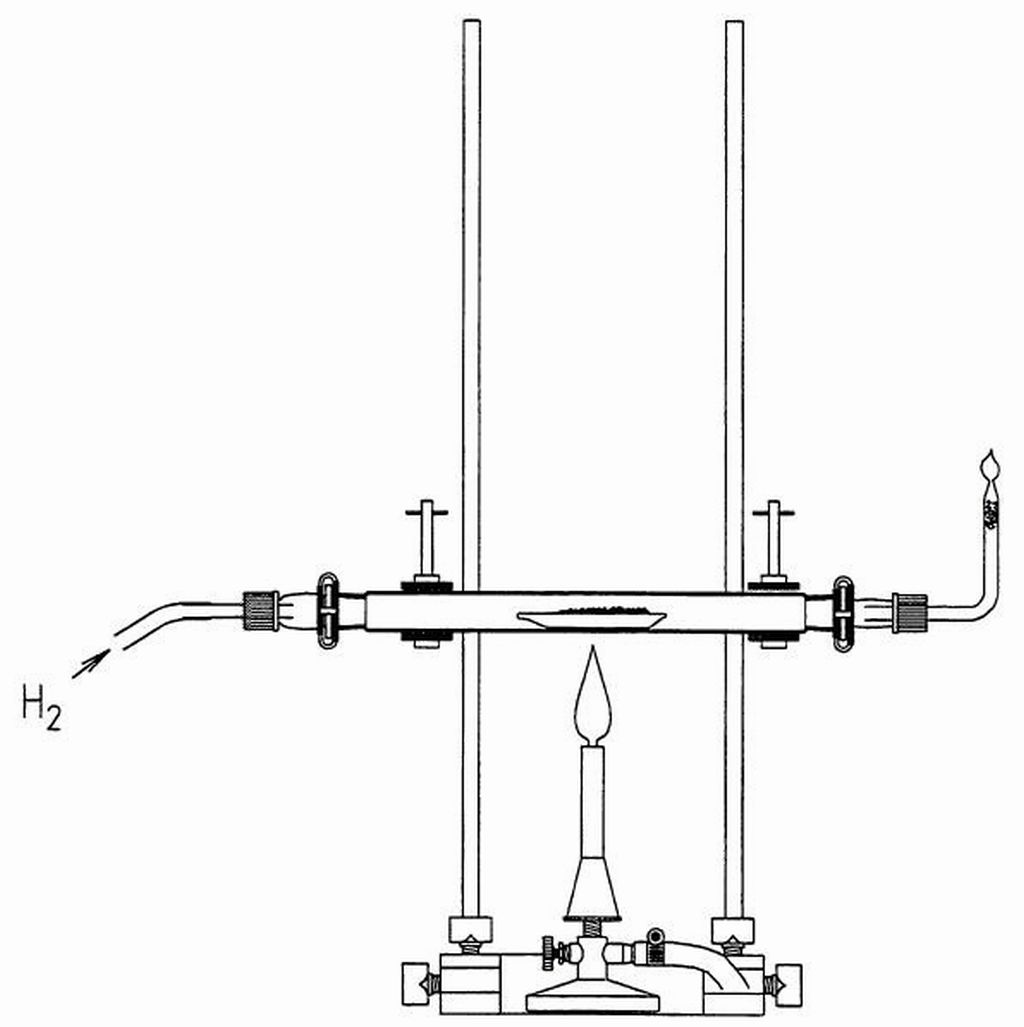
Principle
The reduction, as the reversal of the oxidation, can be achieved thermally or with the aid of a reducing agent.
Some metal oxides can be decomposed into the metal and oxygen under the influence of thermal energy. In the case of less noble metals, a reducing agent is required for obtaining the elements. The redox processes during the preparation of lead demonstrate the relationship between oxidation and reduction.
Benefits
- Stable and safe setup due to solid stand material
Tasks
- Reduction of iron oxide including the formation of hydrogen based on pyrophoric iron.
Learning objectives
- Reduction
- Oxidation
- Redox reaction
- Iron