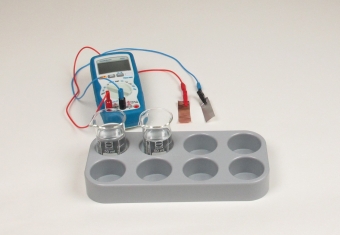
Principle
The first galvanic cell was described by the Italian physicist Alessandro Graf Volta in 1799. This "Volta cell" or "voltaic cell" is also a copper/zinc cell, but in contrast to the Daniell cell, which was developed later, both electrodes are dipped here into the same electrolyte solution (dilute sulphuric acid). This relatively simple construction allowed several such cells to be combined together in a very compact form. Such batteries of voltaic cells became known as "voltaic piles", and were used to generate higher voltages.
Learning objectives
- Principle of a voltaic cell
Benefits
- Easy teaching and efficient learning by using interactive experimentation PHYWE-Software
- Experiment is part of a complete solution set with experiments for the topic Electrochemistry matched with international curriculum: all topics are covered