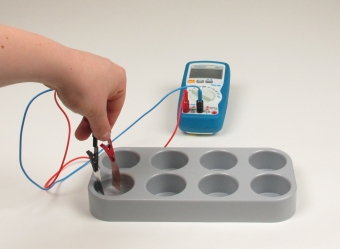
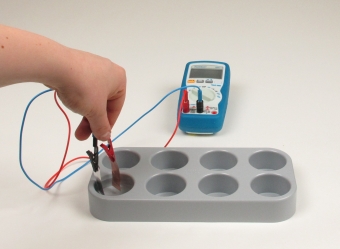
Principle
The metals zinc and copper tend to dissolve in water to a certain extent. This tendency can be designated as "solution pressure". On dissolving, metal atoms on the surface of the metal electrodes pass into the ionic state. In this experiment students realize, that when two electrodes of different metals, e.g. copper and zinc, are dipped into pure distilled water, an electric voltage can be detected between the two metal sheets.
Learning objectives
-
solution pressure (of metall-ions) creates a difference of potential
Benefits
- Easy teaching and efficient learning by using interactive experimentation PHYWE-Software
- Experiment is part of a complete solution set with experiments for the topic Electrochemistry matched with international curriculum: all topics are covered