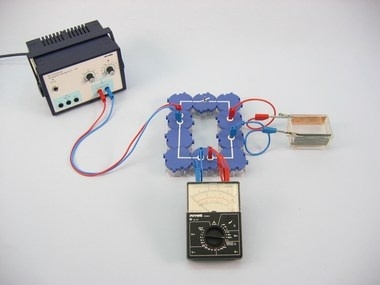
Principle
Items of practical use are frequently coated with a layer of nickel, chromium, silver or gold, for aesthetical reasons or as protection against corrosion. The coating process is carried out electrochemically, and is called galvanisation.
The students are to coat a part of a sheet of iron with a layer of metallic copper in a model experiment.
Benefits
- No additional cable connections between the building blocks needed - clear arragned and quick setup
- Contact saftey due to puzzle blocks system
- Corrosion-free gold plated contacts
- Doubled earning sucess: Electric circuit diagram on top, real components can be seen unterside
Tasks
How can the surface of a base metal be made more noble?
Demonstrate, in a model experiment, how a sheet of iron can be coated with a layer of copper.