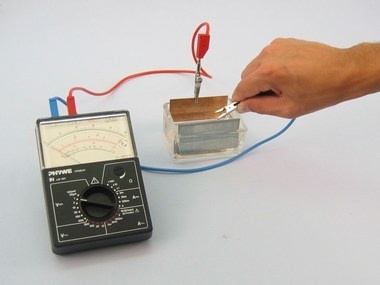
Principle
In principle, a galvanic cell consists of two different metallic electrodes which dip into an aqueous solution of an electrolyte. A voltage is generated between the electrodes, whose origin can be explained, in a simplified way, by the passage of positive metal ions from the surface of the electrodes into the solution. Consequently freely mobile electrons are left behind on the electrodes.
As this process occurs to different extents with different metals, the strengths of their negative charges are different. This difference in charge is the source of the voltage.
In this experiment, the students should become acquainted with the construction and mode of action of single cells which are frequently used in practice, but then are more complicated.
Benefits
- No additional cable connections between the building blocks needed - clear arragned and quick setup
- Contact saftey due to puzzle blocks system
- Corrosion-free gold plated contacts
- Doubled earning sucess: Electric circuit diagram on top, real components can be seen unterside
Tasks
How can electric current be generated from chemical processes?
Examine whether voltages are generated between two electrodes of different materials held in an aqueous solution of an electrolyte.
Necessary accessories
You cannot use the item without these accessories!