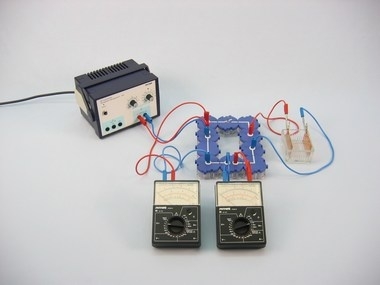
Principle
Salts, acids and bases are electrolytes. In pure form they do not (or hardly) conduct electric current, because in this condition they have no (or only extemely few) freely mobile electrons. In water, the dissolved electrolyte divides (dissociates) into positive and negative ions.
When voltage is applied to two electrodes which are dipped into an aqueous solution of an electrolyte, the ions migrate towards the electrode which has the opposite electric charge. Aqueous solutions of electrolytes are therefore capable of conducting electric current.
The students should find this out in this experiment, and also get to understand why an electrolyte which is not dissolved (or not melted) as well as distilled water are not conductive, or hardly so.
Benefits
- No additional cable connections between the building blocks needed - clear arragned and quick setup
- Contact saftey due to puzzle blocks system
- Corrosion-free gold plated contacts
- Doubled earning sucess: Electric circuit diagram on top, real components can be seen unterside
Tasks
Do liquids also conduct electric current?
You will determine if water containing dissolved substances conducts electric current.