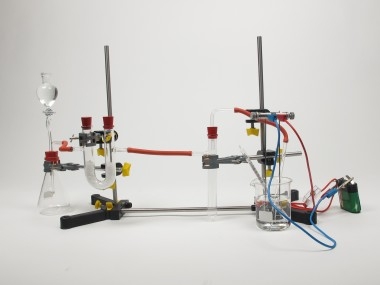
Principle
Ammonia chloride (and household detergents) and sodium hydroxide form a gas smelling like a horse stable - ammonia.
Ammonia shows the typical properties of an alkali only in the form of an aqueous solution. Alkalis behave in a similar way as acids. Ammonium chloride is decomposed by sodium hydroxide to the following equation: NH4Cl + NaOH → NH3 + H2O + NaCl
Learning objectives
- Household detergents contain alkalis
Benefits
- Easy teaching and efficient learning by using interactive experimentation PHYWE-Software
- Experiment is part of a complete solution set with experiments for the topic acids, alkalis and salts matched with international curriculum: all topics are covered