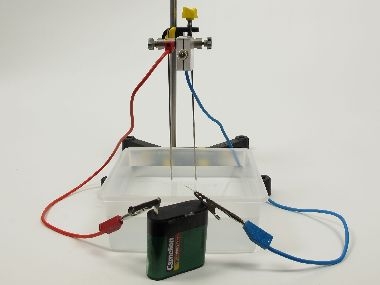
Alkaline solutions can be obtained from areaction of base metals with water. - The formation of the alkalme solution is indicated by the change in conductivity (the filament lamp lights up or starts to glow more intensively) and by the change in colour of the indicator.
Tasks
Prepare an alkaline solution of lithium and study the conductivity during the reaction.
Benefits
- Easy teaching and efficient learning by using interactive experimentation PHYWE-Software
- Experiment is part of a complete solution set with experiments for the topic acids, alkalis and salts matched with international curriculum: all topics are covered