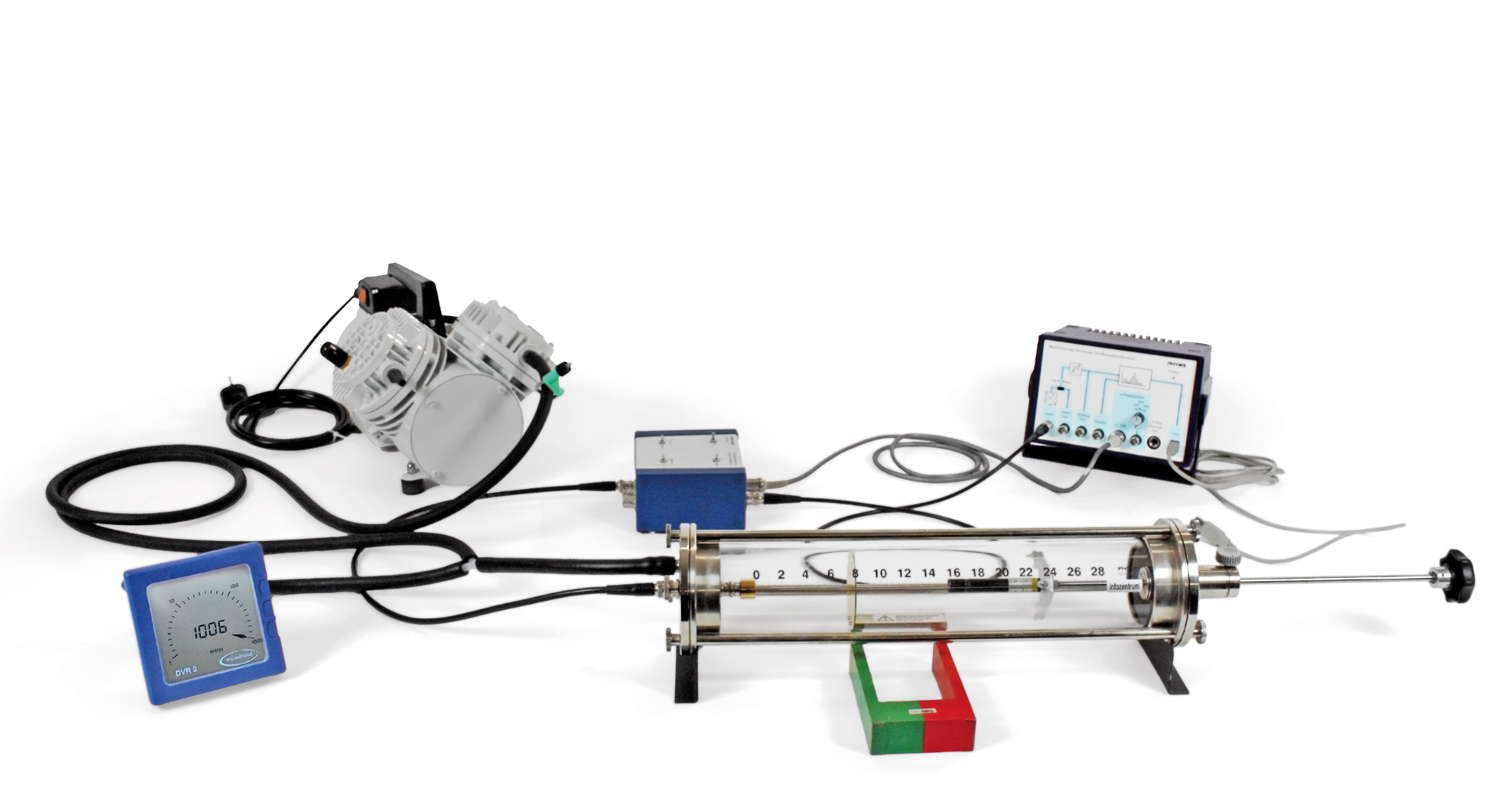
Principle
The relationship between the angle of scattering and the rate of scattering of alpha-particles by gold foil is examined with a semiconductor detector. This detector has a detection probability of 1 for alpha-particles and virtually no zero effect, so that the number of pulses agrees exactly with the number of alpha-particles striking the detector. In order to obtain maximum possible counting rates, a measurement geometry is used which dates back to Chadwick. It is also possible in this case to shift the foil and source in an axial direction (thus deviating from Chadwick's original apparatus), so that the angle of scattering can be varied over a wide range. In addition to the annular diaphragm with gold foil, a second diaphragm with aluminium foil is provided in order to study the influence of the scattering material on the scattering rate.
Benefits
- Experience the essence of the Nobel Prizes: Rutherford (1908)
- Gold and Aluminium foil included
- The combination of multi channel analyzer (MCA), preamplifier and alpha detector leads to precise results
- Transparent glass container with perfect visibility of the whole setup and good vacuum conditions, used in several experiments
Tasks
- The particle rates are measured at different angles of scattering between about 20° and 90°. The measurements are compared with the particle rates calculated by means of the Rutherford theory for the measurement geometry used.
- The particle rates are measured in the case of scattering by aluminium and gold with identical angles of scattering in each case. The ratio of the two particle rates is compared with the particle rate calculated from Rutherford's scattering equation.
Learning objectives
- Scattering
- Angle of scattering
- Impact parameter
- Central force
- Coulomb field
- Coulomb forces
- Rutherford atomic model
- Identity of atomic number and charge on the nucleus
Software included. Computer not provided.