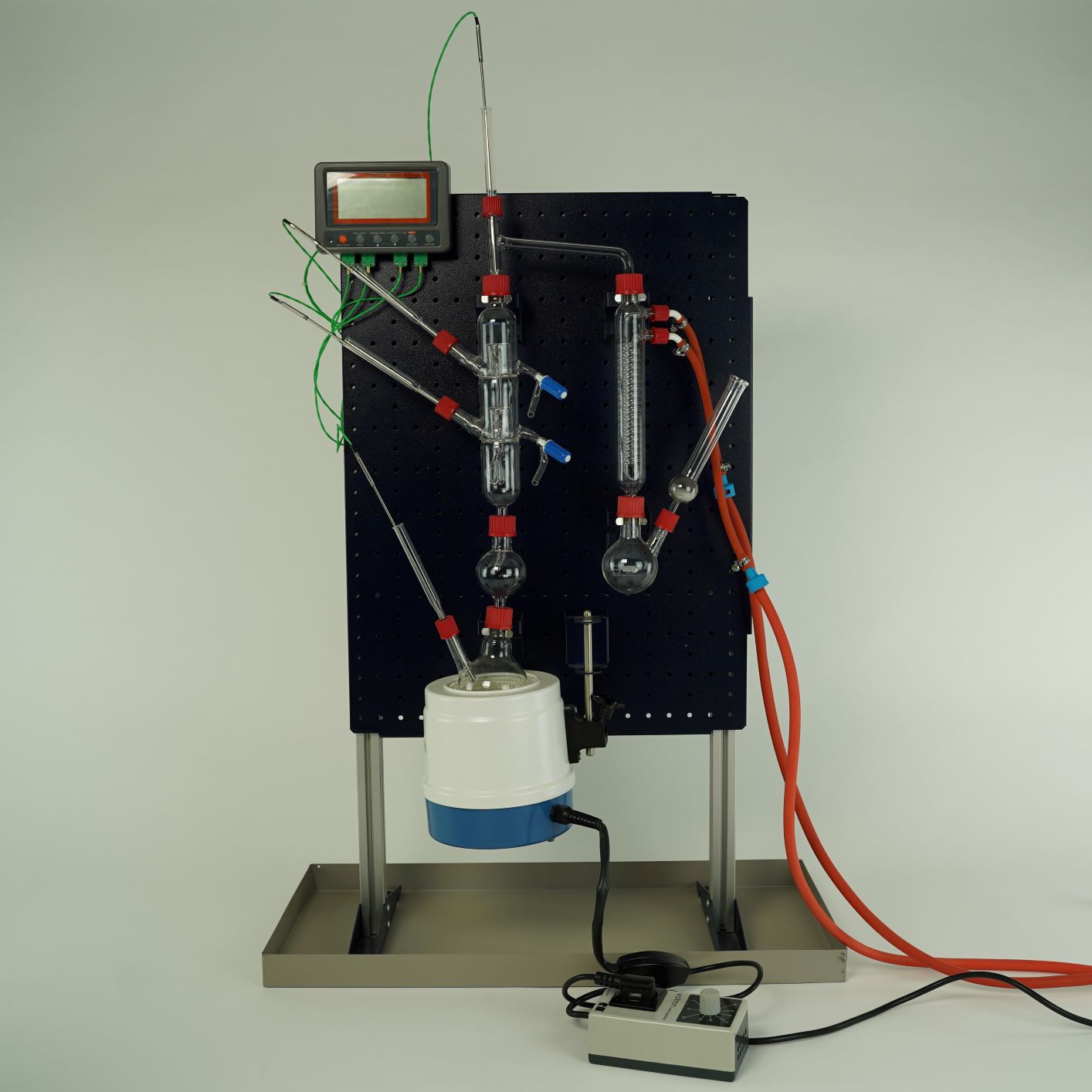
Principle
Crude oil, still one of the most important raw materials in the world, is a mixture of many hydrocarbons. Because the crude oil is a mixture of substances with different boiling temperatures, individual substances with different boiling ranges can be separated by distillation in fractions. In this experiment a model of crude oil is distilled.
Learning objectives
-
Distillative separation of a liquid mixture
-
How a fractional distillation works
- Destillation of cruide oil
- Composition of cruide oil (this experiment examines a model of crude oil)
Benefits
- The glass equipment makes it easy for observers to recognise and understand the experiment set-up.