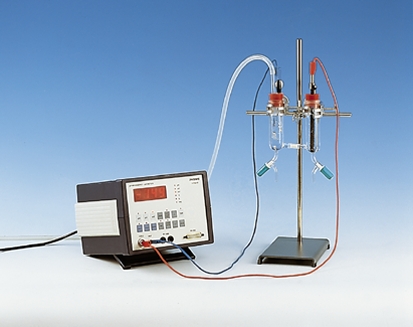
Principle
The redox series is one of the most important tools in "electrochemistry". The redox series arrange metals according to their efforts to emit electrons. The redox series generally begins with the base metals (e.g., iron, zinc) and goes to noble metals (e.g., gold, silver).
In this experiment students examine the electrochemical potential of the metals. For this purpose, hydrochloric acid is reacted with various metals. Students observed that some metals react with the hydrochloric acid (formation of hydrogen) and some metals not. With this experiment results, students can arrange a (simple) redox series with hydrogen as a reference element. In a second experimental part, a simple normal hydrogen electrode is constructed and the corresponding voltage of redox potentials is measured.