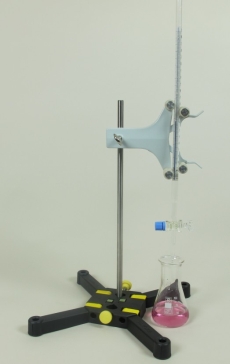
Principle
During this experiment, the students have to determine the unknown concentration of sulphuric acid (solution to be analysed) with the aid of a suitable indicator (here: phenolphthalein). For this purpose, a known volume of the acid is titrated with a volume of a sodium hydroxide solution of a known concentration (standard solution) until the indicator changes colour. The used volume of the standard solution and its concentration are then used to calculate the concentration of the solution being analysed.
Learning objectives
- Titration of sulphuric acid
Benefits
- Experiment literature available for pupils and teachers: Minimum preparation time
- Simple teaching and efficient learning by using the available interactive experiment literature