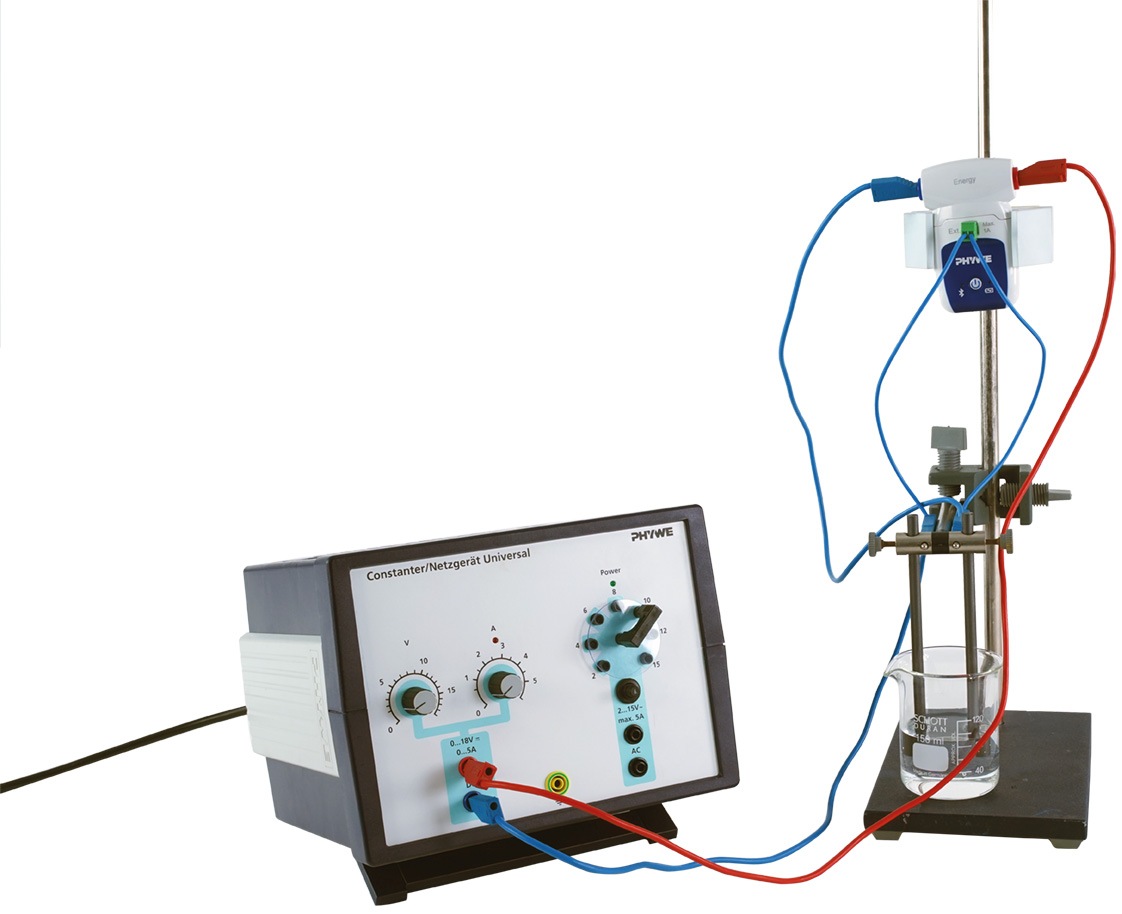
Principle
If the oxidation and reduction steps of an electrode reaction are rapid (high exchange current densities) then the passage of charge across the electrode-solution interface will barely displace the reaction equilibrium. Such an electrode is said to be non-polarisable in the sense that its potential, for small currents, is stable and equal to the equilibrium electrode potential. If, on the other hand, reaction equilibrium is established only slowly due to the kinetic inhibition of a step involved in the electrode reaction, then the electrode is said to be polarisable. To induce the reaction to proceed in a given direction the kinetic inhhibition of the reaction must be overcome by applying a high overpotential. Electrode polarisation and the presence of overpotentials are important concepts in understanding electrode processes. They underlie the fact that galvanic cells always deliver current at less than the equilibrium e.m.f. and that an applied potential greater than the equilibrium e.m.f. is required in order to drive a reaction in an electrolytic cell. Futhermore, a number of important electochemical devices (e.g. the lead-acid accumulator) and electroanalytical techniques (e.g. polarography) make use of the inhibition (high overpotential) of certain electrode reactions.
Benefits
- Simultaneous measurement of current and voltage
- Simplified implementation: all pre-settings already prepared
Tasks
- Record the current-potential curve for the electrolysis of a 1 M hydrochloric acid solution using graphite rod electrodes and determine the decomposition voltage.
- Discuss the physical processes determining the form of this curve.
- By replacing the graphite rod cathode with a series of different metal rod electrodes, compare the overpotentials for hydrogen evolution at these metals.
Learning objectives
- Electrode kinetics
- Polarisation
- Overpotential
- Irreversible processes
- The electrode-electrolyte interface
- Voltammetry and current-potential curves
- Relevance to electrolysis
- Fuel cells
- Corrosion
- Polarography
Software included. Computer not provided.