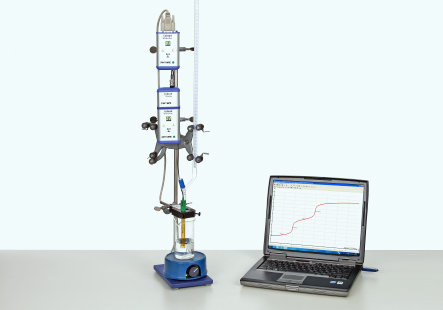
Principle
Phosphoric acid and sodium hydroxide are to be used to give an example of a titration of a polyvalent acid with a strong base.
Benefits
- Drop counter optimizes the experimental procedure
- Simplified implementation: all pre-settings already prepared
- Extensive automation of the experiment allows concentration on the learning objectives
Tasks
- Measure the alteration of the pH value during a titration of an aqueous solution of phosphoric acid with a 0.1 molar sodium hydroxide solution at constant temperature using the Cobra4 system.
- From the titration curve read the equivalence points and pKa values.
- Determine the concentration of the acid.
Learning objectives
- Phosphoric acid
- Sodium hydroxide
- Polyvalent acid
- pKa values
- Proteolysis
- pH value
Necessary accessories
- Precision balance 620g/0.001g